By Aneri Upadhyay
Staff Writer
The United States Food and Drug Administration recently approved an at-home test for flu and Covid-19 on Feb. 24. This test would allow the general public to be able to determine if they have the flu or Covid-19, an important distinction due to the similar symptoms from both illnesses.
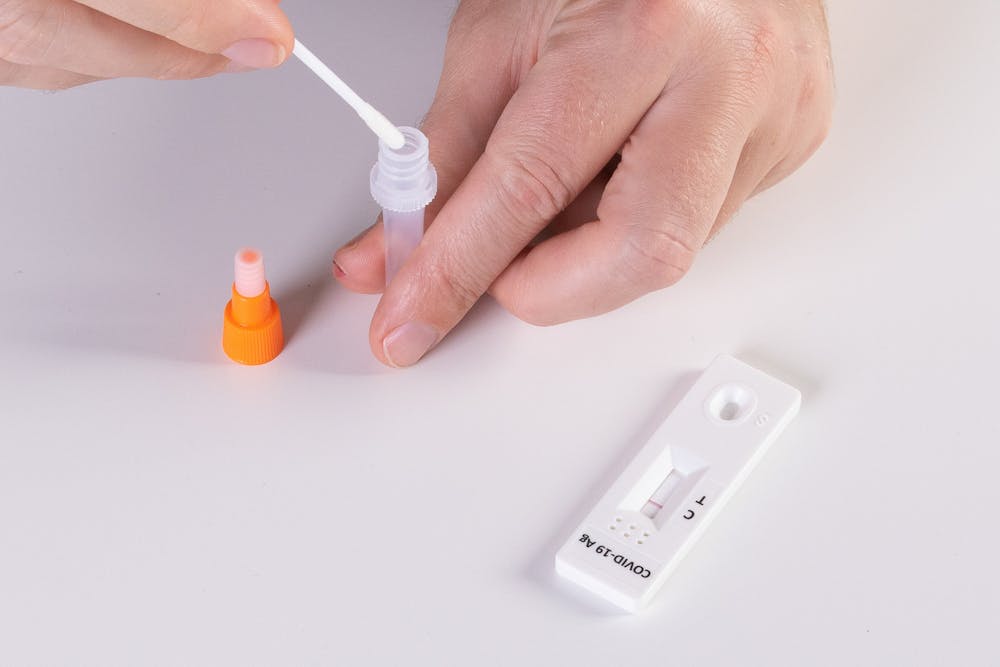
The Lucira Covid-19 & Flu Home Test is an over-the-counter test, according to AP News. It requires individuals to nasal swab themselves in order to get a sample and delivers results in about 30 minutes.
Before this, there has never been an at-home test for influenza or the flu. Now, respiratory symptoms can be diagnosed quicker for faster awareness and treatment.
As reported by the FDA, the test can be used by people aged 14 or older or an adult can swab someone aged 2 or older.
In previous studies, the Lucira test has been proven to correctly diagnose 99.3% of negative influenza and 90.1% of positive influenza samples, along with 100% of negative Covid-19 and 88.3% of positive Covid-19 samples.
Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, called this a “major milestone in bringing greater consumer access to diagnostic tests that can be performed entirely at home.”
Due to the slight chance of receiving false results, it is necessary to still remain cautious and isolated when respiratory symptoms are present. The FDA even offered advice for what to do if the results do not align with the symptoms.
As reported by AP News, the FDA stated that “individuals who test negative and continue to experience symptoms of fever, cough and-or shortness of breath may still have a respiratory infection and should seek follow-up care with their healthcare provider.”
This is not the only healthcare-related breakthrough that Lucira Health has created, according to NPR. It was also the first company to get FDA approval for rapid home tests for Covid-19.
The need for these tests are even more apparent after last fall, when there was an increase in both positive Covid-19 results and the flu, along with respiratory syncytial virus. These rates have since decreased.
As reported by the New York Times, it is unknown when exactly the test will become available to the general public. This is especially relevant with the Biden Administration’s desire to stop the Covid-19 public health emergency, which could raise prices for over-the-counter medication for people with insurance. People on Medicare and private insurance would also need to pay for future tests rather than receiving eight at-home tests for free every month.
The emergence of at-home tests to distinguish influenza from Covid-19 is especially important with the removal of on campus testing at the College. Without the accessibility of going to Decker Hall to get tested for Covid-19, students are left with the unknown possibility of having either Covid-19 or the flu. Having over-the-counter tests could eliminate this unnecessary worry and provide clarity at a time of sickness.